Ustilaginomycotina
The true smut fungi
Robert Bauer, Dominik Begerow, and Franz Oberwinkler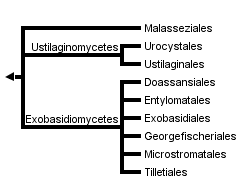

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxNote: The classification of Ustilaginomycotina is being revised. The tree at the top of this page reflects the current classification, whereas the following tree and the text on this page reflect a prior classification.

Introduction
The class Ustilaginomycetes comprises more than 1400 species of basidiomycetous plant parasites, which are distributed in approximately 70 genera. They occur throughout the world, although many species are restricted to tropical, temperate or arctic regions. Some species of Ustilago and Tilletia, e.g. the barley, wheat or maize smut fungi, are well known because they are of economic importance. For example, from 1983 to 1988 the barley smut fungi reduced annual yields by 0.7% to 1.6% in the prairie provinces in central Canada, causing average annual losses of about U.S. $8,000,000 (Thomas 1989). Tilletia contraversa is important in the international wheat trade (Trione 1982), and 2-5% of the plants in a corn field are generally infected by Ustilago maydis, while up to 80% of a field can be infected if conditions are good for the smut fungus. On the other hand, the galls of U. maydis are considered a delicacy in the Mesoamerican tradition. They are known in Mexico as "Huitlacoche" and in the U.S.A. as "maize mushroom", "Mexican truffles" or "caviar azteca" (Valverde et al. 1995).
Characteristics
- Plant parasitism
- Cellular interaction with primary interactive vesicles
- Cell wall carbohydrate composition with dominance of glucose and absence of xylose
- 5S rRNA secondary structure of type B
- Septal pores without parenthesomes, but in most cases with distinctive tripartite membrane caps or discs
- Life cycle with a parasitic dikaryophase and a saprobic haplophase
Plant parasitism
In contrast with the Urediniomycetes and Hymenomycetes, the Ustilaginomycetes are ecologically well characterized by their parasitism of vascular plants. Two of the more than 1400 species live on lycophytes, one on ferns, two on conifers, whereas all other Ustilaginomycetes parasitize angiosperms with about 810 species on Poaceae and 170 on Cyperaceae. Interestingly, no species has been reported to parasitize Orchidaceae although this family, with about 20,000 species, is one of the largest groups of the angiosperms. With a few exceptions the teliospore-forming species of the Ustilaginomycetes parasitize nonwoody herbs, whereas those without teliospores prefer woody trees or bushes. However, almost all species sporulate on or in parenchymatic tissues of the hosts. Depending upon the species the sori appear in different organs of the hosts, e.g. in roots, stems, leaves, inflorescences, flowers, anthers, ovaries, seeds etc.
Cellular interaction
An important apomorphy for the Ustilaginomycetes is the presence of zones of host-parasite interaction with fungal deposits resulting from exocytosis of primary interactive vesicles (Bauer et al. 1995a, 1997).

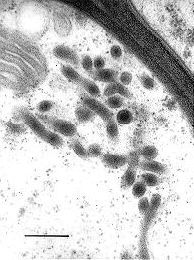
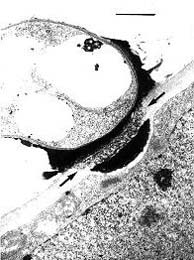
Fig.1. Transmission electron micrograph showing primary interactive vesicles in Exobasidium pachysporum. Scale bar = 0.2 µm. © R. Bauer 1997
Fig. 2. Transmission electron micrograph showing a transfer stage between Mycosyrinx cissi (upper cell) and its host (lower cell). Note the infiltrated host cell wall (between the two arrows) and the deposit at the host cell. Scale bar = 1 µm. © R. Bauer 1997
The contents of these vesicles (Fig. 1) are transferred to the host plasma membrane (Fig. 2). Two major types are recognized. (i) Local interaction zones (Fig. 3): short-term production of primary interactive vesicles per interaction site results in local interaction zones, and (ii) enlarged interaction zones (Fig. 4): continuous production and exocytosis of primary interactive vesicles results in the continuous deposition of fungal material at the whole contact area with the host cell.

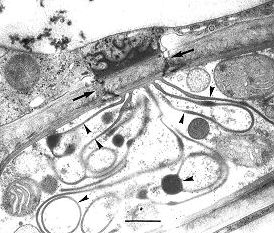
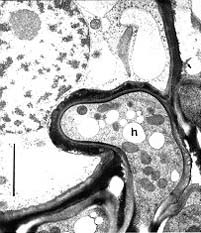
Fig. 3. Transmission electron micrograph showing a local interaction zone (arrows) between Exobasidium pachysporum (lower cell) and its host (upper cell). Note the interaction apparatus (arrowheads) and the deposit at the host cell. Scale bar = 0.5 µm. © R. Bauer 1997
Fig. 4. Transmission electron micrograph showing an enlarged interaction zone between Ustacystis waldsteiniae and its host. The haustorium (h) is encased by electron-opaque material. Scale bar = 2 µm. © R. Bauer 1997
Cell wall carbohydrate composition
Prillinger et al. (1993) distinguished between several types of cell wall carbohydrate composition within the Basidiomycota. The Ustilaginomycetes have a distinctive type with dominance of glucose and absence of xylose that separates them from the Urediniomycetes and Hymenomycetes.
5S rRNA
Gottschalk and Blanz (1985) distinguished between a type A and a type B secondary structure of the 5S rRNA. The Ustilaginomycetes share the type B secondary structure with the Hymenomycetes.
Septal pores
In contrast with the Hymenomycetes, the septal pores of the Ustilaginomycetes are without multilayered parenthesomes. In contrast with the Urediniomycetes, in most Ustilaginomycetes the septal pores are enclosed by distinctive, tripartite membrane caps or discs (Bauer et al. 1995b, Bauer et al. 1997, Fig. 5).

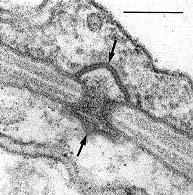
Fig. 5. Transmission electron micrograph showing a typical septal pore apparatus of the Ustilaginomycetes (Entyloma callitrichis) with two membrane caps (arrows). Scale bar = 0.1 µm. © R. Bauer 1997
Life cycle
The Ustilaginomycetes present a rather uniform life cycle with a saprobic haploid phase and a parasitic dikaryophase (e.g. Sampson 1939; Fig. 6). The haploid phase usually commences with the formation of basidiospores after meiosis of the diploid nucleus in the basidium and ends with the conjugation of compatible haploid cells to produce dikaryotic, parasitic mycelia. The dikaryotic phase ends with the production of basidia. In the majority of the Ustilaginomycetes the young basidium becomes a thick-walled teliospore and separates at maturity from the sorus, thus functioning as a dispersal unit. Most of the Ustilaginomycetes are dimorphic, producing a yeast or yeast-like phase in the haploid state. Almost all Ustilaginomycetes multiply mitotically in the saprobic phase, either with yeasts or with ballistoconidia, or with both.

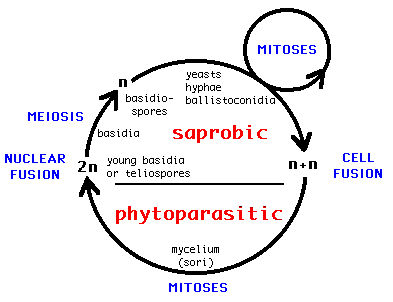
Fig. 6. Generalized life cycle of the Ustilaginomycetes. © R. Bauer and F. Oberwinkler 1997
The Ustilaginomycetes share most characteristics of the life cycle with the Microbotryales, which traditionally were considered belonging to the Ustilago-group. However, several independent characters show that the microbotryaceous species of the genera Aurantiosporium, Fulvisporium, Liroa, Microbotryum, Sphacelotheca, Ustilentyloma and Zundeliomyces are actually Urediniomycetes (Gottschalk and Blanz 1985, Prillinger et al. 1993, Swann and Taylor 1993, Bauer et al. 1997).
Discussion of Phylogenetic Relationships
Since Tulasne and Tulasne (1847) the smut fungi are traditionally divided into the phragmobasidiate Ustilaginaceae and the holobasidiate Tilletiaceae, which are sometimes treated as separate orders (Kreisel 1969, Oberwinkler 1987). Difficulties in their classification have been discussed, e.g. by Durán (1973) and Vánky (1987), neither of whom listed higher taxa in this group. Based predominantly on host-parasite interactions and septal pore apparatus, a radical change in the systematics of smut fungi has been proposed by Bauer et al. (1997). As a result, on the one hand the Microbotryales were excluded from the Ustilaginomycetes and on the other the Exobasidiales s. l., Graphiolales and Microstromatales were included in this group. Cellular interaction (Bauer et al. 1997) and cell wall carbohydrate composition (Prillinger et al. 1993) indicate that the class Ustilaginomycetes in this composition is monophyletic, and phylogenetic analyses of rDNA sequences (Swann and Taylor 1993, Berres et al. 1995, Begerow et al. 1997) are consistent with this hypothesis.
Ultrastructural and rDNA sequence analyses provide evidence for the existence of three major groups within the Ustilaginomycetes (Bauer et al. 1997, Begerow et al. 1997). The basal dichotomy is between the Entorrhizomycetidae and the branch uniting the Ustilaginomycetidae and Exobasidiomycetidae (Bauer et al. 1997, Begerow et al. 1997). In contrast with the Ustilaginomycetidae and Exobasidiomycetidae, the septal pores of the Entorrhizomycetidae are not enclosed by tripartite membrane caps. The Ustilaginomycetidae form enlarged interaction zones, whereas the Exobasidiomycetidae form local interaction zones.
The Ustilaginomycetes represents the sister group of the Hymenomycetes. Type B secondary structure of the 5S rRNA and glucose as major cell wall carbohydrate component are shared by both classes (Gottschalk and Blanz 1985, Prillinger et al. 1993).
Subgroups of Ustilaginomycetes
Lack of membrane bands or caps at the pores and the presence of local interaction zones without interaction apparatus characterize the Entorrhizomycetidae (Bauer et al. 1997). Entorrhiza is the single genus currently identified of this group.
Presence of enlarged interaction zones characterizes the Ustilaginomycetidae (Bauer et al. 1997). This statistically well-supported subclass (Begerow et al. 1997) comprises 33 teleomorphic (with a known sexual stage) and one anamorphic (without a known sexual stage) genera, e.g. Anthracoidea living on Cyperaceae, Cintractia living on Cyperaceae and Juncaceae, Doassansiopsis living on mono- and dicots, Farysia living on Cyperaceae, Melanotaenium s. str. living on dicots, Mycosyrinx living on Vitaceae, Pseudozyma (anamorphic genus), Sporisorium living on Poaceae, Thecaphora living on dicots, Urocystis living on mono- and dicots or Ustilago s.str. mainly living on Poaceae.
The Exobasidiomycetidae differ from the Ustilaginomycetidae by forming local interaction zones and from the Entorrhizomycetidae by having membrane caps at the pores (Bauer et al. 1997). This subclass contains 35 teleomorphic and two anamorphic genera, e.g. Botryoconis living on Lauraceae, Brachybasidium living on Arecaceae, Coniodictyum living on Rhamnaceae, Doassansia living on mono- and dicots, Entyloma living on dicots, Exobasidium living on dicots, Georgefischeria living on Convolvulaceae, Graphiola living on Arecaceae, Malassezia (anamorphic genus), Microstroma living on Juglandaceae and Fagaceae, Tilletia living on Poaceae, Tilletiaria (only known in laboratory) or Tilletiopsis (anamorphic genus).
The term smut fungus
Like the terms agaric, polypore, lichen etc. the term smut fungus circumscribes the organization and life strategy of a fungus, but it is not a taxonomic term. Fungi that look superficially similar to the teliospore-forming members of the Ustilaginomycetes evolved in different fungal groups, e.g. the Microbotryales in the Uredinomycetes (Bauer et al. 1997) or Schroeteria in the Ascomycota (Nagler et al. 1989).
References
Bauer, R., Mendgen, K. and Oberwinkler, F. 1995a. Cellular interaction of the smut fungus Ustacystis waldsteiniae. Can. J. Bot. 73:867-883.
Bauer, R., Mendgen, K., and Oberwinkler, F. 1995b. Septal pore apparatus of the smut Ustacystis waldsteiniae. Mycologia 87:18-24.
Bauer, R., Oberwinkler, F. and Vánky, K. 1997. Ultrastructural markers and systematics in smut fungi and allied taxa. Can. J. Bot. 75:1273-1314
Begerow, D., Bauer, R. and Oberwinkler, F. 1997. Phylogenetic studies on nuclear large subunit ribosomal DNA sequences of smut fungi and related taxa. Can. J. Bot. 75:(appears in Dec.)
Berres, M.A., Szabo, L.J. and McLaughlin, D.J. 1995. Phylogenetic relationships in auriculariaceous basidiomycetes based on 25S ribosomal DNA sequences. Mycologia 87:821-840.
Durán, R. 1973. Ustilaginales. In: Ainsworth, G.C., Sparrow, F. K. and Sussman, A.S. (eds.) The fungi, vol 4B. Academic Press, New York, London, pp 281-300.
Gottschalk, M. and Blanz, P.A. 1985. Untersuchungen an 5S ribosomalen Ribonucleinsäuren als Beitrag zur Klärung von Systematik und Phylogenie der Basidiomyceten. Z. Mycol. 51:205-243.
Kreisel, H. 1969. Grundzüge eines natürlichen Systems der Pilze. Cramer Verlag, Lehre.
Nagler, A., Bauer, R., Berbee, M., Vánky, K. and Oberwinkler, F. 1989. Light and electron microscopic studies of Schroeteria delastrina and S. poeltii. Mycologia 81:884-895.
Oberwinkler, F. 1987. Heterobasidiomycetes with ontogenetic yeast stages-systematic and phylogenetic aspects. Stud. Mycol. 30:61-74.
Prillinger, H., Oberwinkler, F., Umile, C., Tlachac, K., Bauer, R., Dörfler, C. and Taufratzhofer, E. 1993. Analysis of cell wall carbohydrates (neutral sugars) from ascomycetous and basidiomycetous yeasts with and without derivatization. J. Gen. Appl. Microbiol. 39:1-34.
Sampson, K. 1939. Life cycles of smut fungi. Trans. Br. Mycol. Soc. 23:1-23.
Swann, E.C. and Taylor, J.W. 1993. Higher taxa of basidiomycetes: an 18S rRNA gene perspective. Mycologia 85:923-936.
Thomas, P.L. 1989. Barley smuts in the prairie provinces of Canada, 1983-1988. Can. J. Phytopath. 11:133-136.
Trione, E.J. 1982. Dwarf bunt of wheat and its importance in international wheat trade. Plant Disease 66:1083-1088.
Tulasne, L. and Tulasne, C. 1847. Mémoire sur les Ustilaginées comparées Uredinées. Ann. Sci. Nat. Bot. 3:12-127.
Valverde, M.E., Paredes-Lópes, O., Pataky, J.K. and Guevara-Lara, F. 1995. Huitlacoche (Ustilago maydis) as a food source-biology, composition, and production. CRC Crit. Rev. Food Sci. Nutr. 35:191-229.
Vánky, K. 1987. Illustrated genera of smut fungi. Cryptogamic Studies 1:1-159.
Title Illustrations

| Scientific Name | Entorrhiza casparyana, Juncus articulatus |
|---|---|
| Comments | Galls on the roots of Juncus articulatus induced by Entorrhiza casparyana |
| Image Use |
 This media file is licensed under the Creative Commons Attribution License - Version 3.0. This media file is licensed under the Creative Commons Attribution License - Version 3.0.
|
| Copyright |
© 1997 Robert Bauer

|
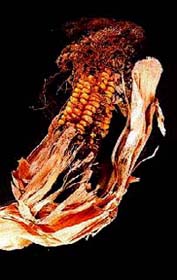
| Scientific Name | Ustilago maydis, Zea mays |
|---|---|
| Comments | Corn galls on Zea mays induced by Ustilago maydis |
| Image Use |
 This media file is licensed under the Creative Commons Attribution License - Version 3.0. This media file is licensed under the Creative Commons Attribution License - Version 3.0.
|
| Copyright |
© 1997 Robert Bauer

|
| Scientific Name | Exobasidium vaccinii, Vaccinium vitis-idaea |
|---|---|
| Comments | Sporulation of Exobasidium vaccinii on Vaccinium vitis-idaea |
| Image Use |
 This media file is licensed under the Creative Commons Attribution License - Version 3.0. This media file is licensed under the Creative Commons Attribution License - Version 3.0.
|
| Copyright |
© 1997 Robert Bauer

|
About This Page
Many thanks to Dr. Meike Piepenbring, Dr. José P. Sampaio and Michael Weiß for their helpful comments.
Robert Bauer

Universität Tübingen, Tübingen, Germany

Ruhr-University Bochum, Germany
Franz Oberwinkler

Universität Tübingen, Germany
Correspondence regarding this page should be directed to Robert Bauer at
Page copyright © 1997 Robert Bauer, , and Franz Oberwinkler
 Page: Tree of Life
Ustilaginomycotina . The true smut fungi.
Authored by
Robert Bauer, Dominik Begerow, and Franz Oberwinkler.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Ustilaginomycotina . The true smut fungi.
Authored by
Robert Bauer, Dominik Begerow, and Franz Oberwinkler.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 26 November 1997
- Content changed 23 January 2008
Citing this page:
Bauer, Robert, Dominik Begerow, and Franz Oberwinkler. 2008. Ustilaginomycotina . The true smut fungi. Version 23 January 2008 (under construction). http://tolweb.org/Ustilaginomycotina/20530/2008.01.23 in The Tree of Life Web Project, http://tolweb.org/









 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site